Canon’s Medical Division Developing a Rapid Diagnostic Test for COVID-19
![]()
Canon USA has jumped into the battle against the novel coronavirus, announcing that the company’s medical division (Canon Medical Systems Corporation) has started “development of a rapid genetic testing system for the novel coronavirus.”
Almost every major camera maker has a medical division, and as the imaging divisions grind to a halt, the medical businesses are ramping up to help the world diagnose and treat COVID-19, the disease caused by the novel coronavirus. Earlier this week, we shared how Fujifilm stock had soared after one of their antivirals showed promising results against COVID-19 in China.
But as important as finding a treatment is increasing our capacity to test for the disease as widely as possible. That’s where Canon is hoping to contribute.
In a press release published by Canon Inc.—the digital imaging division—the company announced that Canon Medical is part of a research project that is working on a rapid test for COVID-19. The test is based on the LAMP method, a testing methodology that “allows for detection of the virus to be performed more easily and quickly” than the current real-time PCR-based tests, and “makes it suitable for testing in local areas where infection is prevalent.”
Canon Inc.’s stock price jumped a little over 7% on the news:
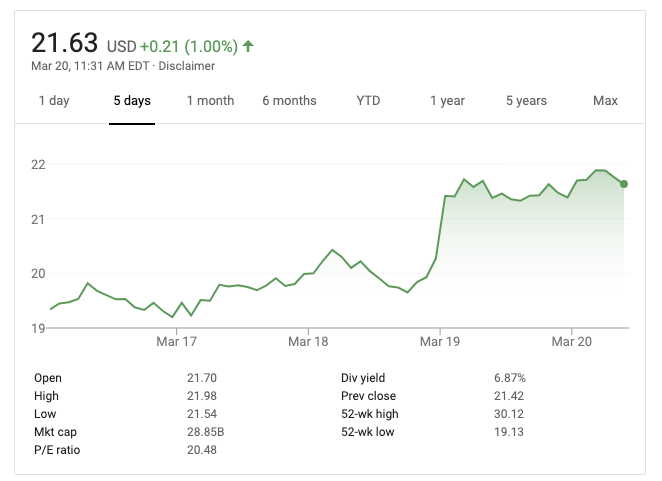
The Canon Medical division has previously developed and provided rapid test kits for both Ebola and Zika during outbreaks in 2015, 2018, and 2019, so this isn’t their first go at such a test.
As with the Fujifilm news, we realize that this isn’t directly associated with the imaging division in general or cameras in particular, but it will certainly contribute to the entire company staying economically healthy during these uncertain times.